About Research
Our research aims to transform prostate cancer detection and management using advanced AI. By integrating diverse data sources, we develop precise diagnostic tools that address the limitations of current methods, ensuring personalized and effective treatment plans for improved patient outcomes.
Background
Currently, the detection of prostate cancer (PCa) relies primarily on serum prostate-specific antigen (PSA) level and digital rectal examination (DRE).
The outcome of both tests is further confirmed with amprostate biopsy as a definite tool for the diagnosis of PCa. However, each of these measures has shortfalls, as prostate biopsy is a painful and unpleasant procedure related to potential complications, while PSA and DRE have very low diagnostic performance, frequently leading to overdetection and overtreatment of clinically insignificant cancer.
In an effort to reduce overtreatment and as an alternative to immediate therapy, guidelines have recommended AS for ~45% of PCa patients who are at low risk for progression, bearing so-called insignificant PCa.
The outcome of both tests is further confirmed with amprostate biopsy as a definite tool for the diagnosis of PCa. However, each of these measures has shortfalls, as prostate biopsy is a painful and unpleasant procedure related to potential complications, while PSA and DRE have very low diagnostic performance, frequently leading to overdetection and overtreatment of clinically insignificant cancer.
In an effort to reduce overtreatment and as an alternative to immediate therapy, guidelines have recommended AS for ~45% of PCa patients who are at low risk for progression, bearing so-called insignificant PCa.
Clinical guidelines have recently adopted multiparametric Magnetic Resonance Imaging (mpMRI) followed by biopsy. Nevertheless, various studies have shown that the biopsy-assigned grade is not correct in >40% of cases. While increasing use of MRI-guided biopsy leads to more PCa being detected in general, it does not improve concordance with the true grade.
Due to how grades are assigned, both under and over-estimation of the true grade are possible – both of which can lead to uncertainty when recommending a treatment. Thus, mpMRI alone does not improve on currently problematic patient management.
Non-invasive urine-based tests (“liquid biopsy”), among others, based on CE-MS technology and marketed by MOS (PCa Status test) have been investigated and proven to have a significant added diagnostic value in detecting clinically significant PCa. Yet, these biomarker solutions are limited by their performance reaching 80% at maximum, not enough to be implemented in the clinical guidelines. At the same time, none of the biomarker solutions currently integrates mpMRI imaging data.
Pilot data show a complementary value of multi-source data in overcoming PCa heterogeneity and thus increasing diagnostic/ prognostic performance. This leaves a huge gap in European clinical practice and a big market (~400,000 PCa patients initially diagnosed annually and frequently monitored thereafter) for an improved diagnostic/ stratification test to be implemented.
Due to how grades are assigned, both under and over-estimation of the true grade are possible – both of which can lead to uncertainty when recommending a treatment. Thus, mpMRI alone does not improve on currently problematic patient management.
Non-invasive urine-based tests (“liquid biopsy”), among others, based on CE-MS technology and marketed by MOS (PCa Status test) have been investigated and proven to have a significant added diagnostic value in detecting clinically significant PCa. Yet, these biomarker solutions are limited by their performance reaching 80% at maximum, not enough to be implemented in the clinical guidelines. At the same time, none of the biomarker solutions currently integrates mpMRI imaging data.
Pilot data show a complementary value of multi-source data in overcoming PCa heterogeneity and thus increasing diagnostic/ prognostic performance. This leaves a huge gap in European clinical practice and a big market (~400,000 PCa patients initially diagnosed annually and frequently monitored thereafter) for an improved diagnostic/ stratification test to be implemented.
Research Data and Visual Analysis
Explore our detailed research data through visual representations, highlighting key findings and advancements in prostate cancer diagnostics. Each figure and its accompanying description offer valuable insights into the data and technologies shaping the future of prostate cancer diagnosis and treatment through ProSTRAT-AI.
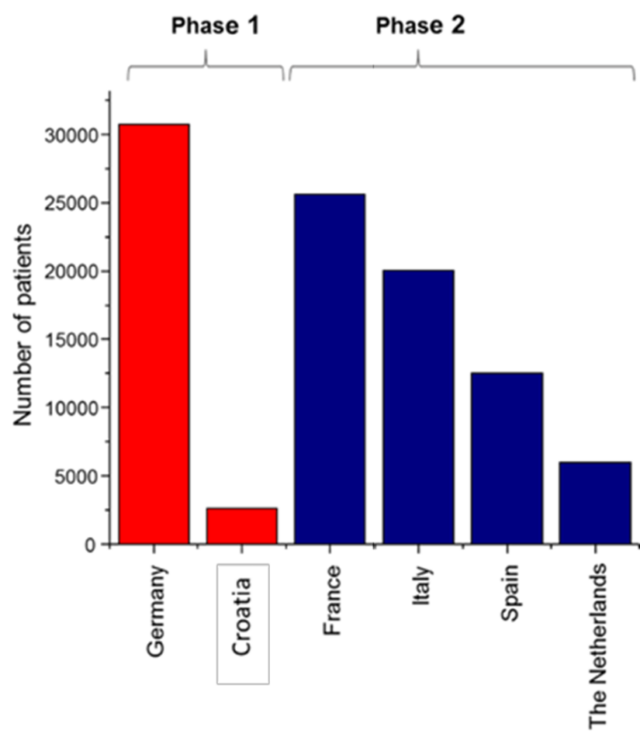
Overview of the target market size in European Union
The key markets were defined based on the number of PCa cases in the population (5-year prevalence). Patients who are initially diagnosed with PCa of Gleason Score <7 and undergo AS represent 45% of all newly diagnosed PCa patients per year and are screened by-annually (target market).
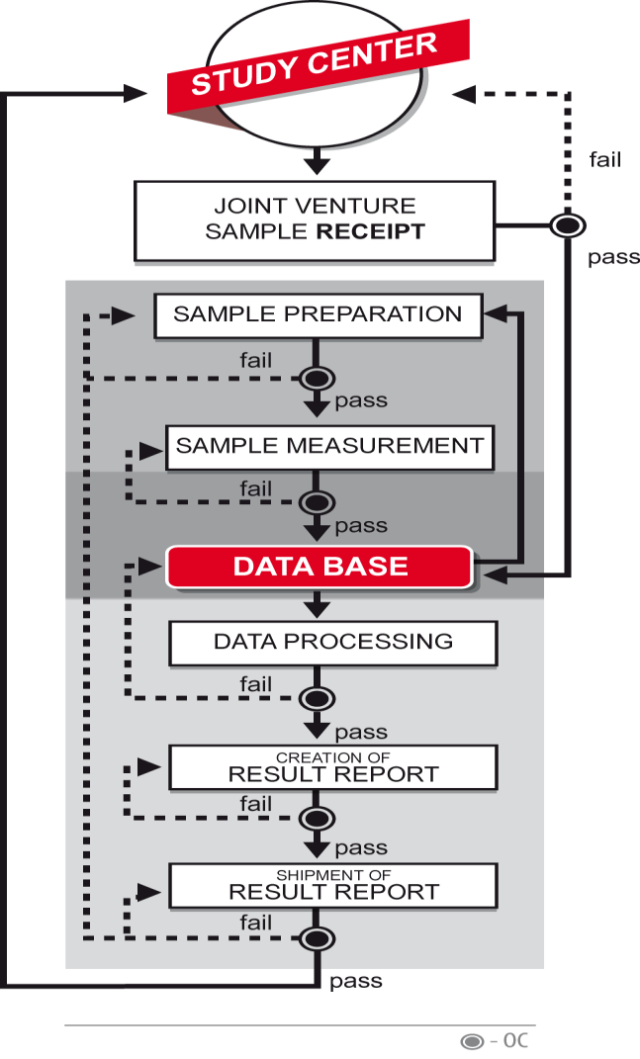
Flowchart for MOS established laboratory quality management system for CE-MS based products
The system covers all steps necessary for applying the prototype in patient care and/or large clinical trials, i.e. each step from sample receipt to result shipment. The system will also easily allow considering clinical research organizations when necessary.
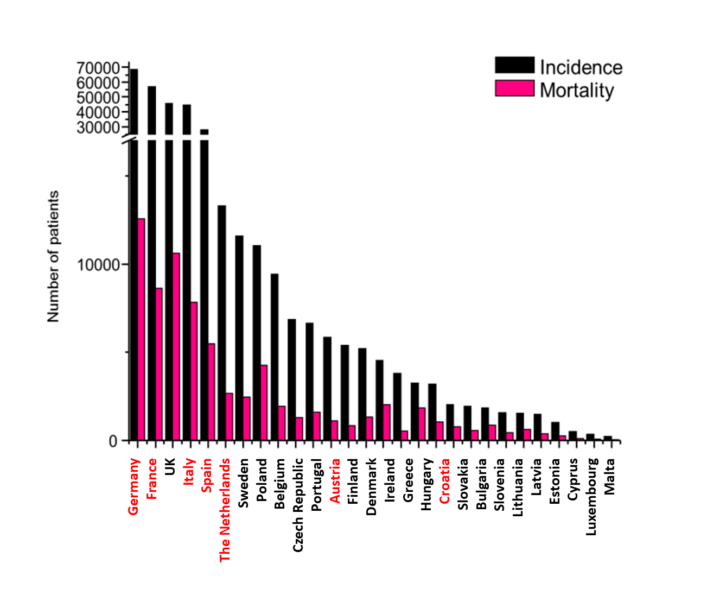
Overview of the incidence (black) and mortality (pink) of PCa in European Countries
With red font the targeted markets for ProSTRAT-AI.
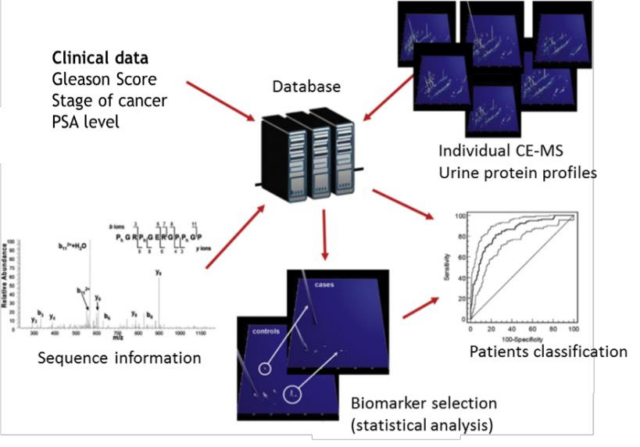
CE-MS based urinary proteome analysis
All urine proteome profiles and matching clinical data are stored in an internal MOS’ database together with the peptide sequences. This allows sample selection and differential proteomic profiling for the purpose of biomarker discovery and patient classification. Furthermore, powerful statistical analysis is feasible on this data.
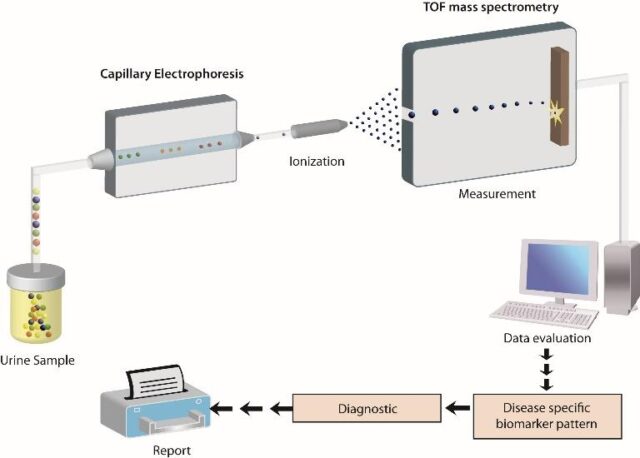
Schematic depiction of the CE-MS analytical and diagnostic workflow
The software solutions developed by MOS enable automatic decoding of the proteomics data and the subsequent construction of a comprehensive, high-dimensional polypeptide pattern specific for the disease under investigation. All polypeptide patterns are stored in a central database as normalized and calibrated datasets. There, they can be directly compared with other existing datasets (controls or indicative of other diseases), taking advantage of the data compatibility due to the same procedure applied in each case and controlled by stringent quality checkpoints.
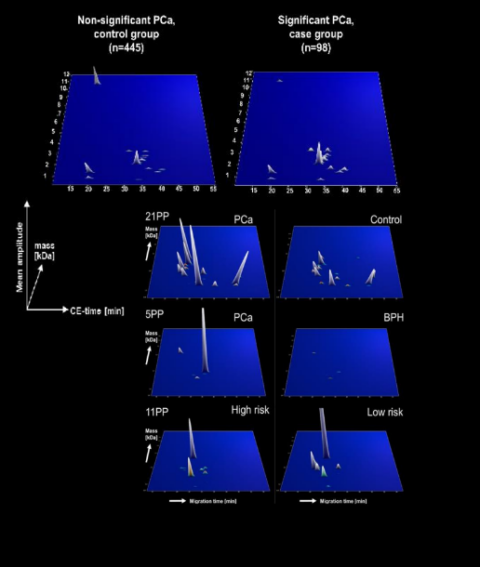
Diagnostic potential of CE-MS based biomarkers.
CE-MS based biomarkers panel have been previously published for diagnosis of prostate cancer and validated in a blinded prospective study7. In a follow-up study, a second validation was performed along with a cost-effectiveness evaluation, confirming the diagnostic accuracy and cost-effectiveness of the developed test5. CE-MS was also used to identify urinary peptides that are significantly associated with a PCa Gleason score ≥7 and thus potentially enable non-invasive risk assessment. Independent validation of these biomarkers resulted in an accuracy of 81%.